Home > Events >Sterile Injection Flexible Manufacturing
Sterile Injection Flexible Manufacturing
In the global sterile injection manufacturing field, Europe, the United States, Japan and other high regulatory markets are featured with the increasing development of flexible manufacturing model with the rise of bio-pharmaceuticals - the basic characteristics include: high value, low yield, automation, complete isolation, flexible manufacturing, the use of pre sterilization parts and the application of disposable technique.
Flexible manufacturing is the direction of the development of future high-end pharmaceutical plants, which not only refers to certain equipment or workshop, but covers all aspects from the pharmaceutical plant construction to equipment manufacturing and software engineering. The features of flexible manufacturing are mainly reflected in the "Modular design concept", "Easy-to-expand production mode", and "Reproducible best effect"
This article analyzes the specific manifestation of flexible manufacturing concept in the construction process of the current high-end sterile injection pharmaceutical plants from the three aspects: pharmaceutical plant engineering construction, equipment manufacturing and software engineering.
01
Pharmaceutical Plant Project Construction
The production of sterile injections, especially biopharmaceuticals, tends to focus on drug development phase. To allow the research and development of drugs entering into the clinical test phase as soon as possible and shorten the time for drug approval and launch to the market, pharmaceutical plants hope that the construction period can be shortened in the project construction phase.
However, the construction of sterile injection pharmaceutical plant is different from that of the general plant. It requires compliance with the GMP requirements, and the reflection of the concept of sterility from all aspects of the plant. To shorten the construction cycle, some pharmaceutical manufacturers began to consider the use of modular plant design concept, and successfully applied it to the actual project.
Such modular plant construction adopts the "Container" build mode. Pharmaceutical manufacturers put forward specific requirements, plant manufacturers divide the workshop of the pharmaceutical plant into multiple modules according to its requirements. These independent modules can be manufactured at the same time, and in the manufacturing phase, the pharmaceutical plant does not need to provide land. These room modules are transported directly to the target site of the pharmaceutical plant for direct setup after they are completed at the manufacturer's plant.
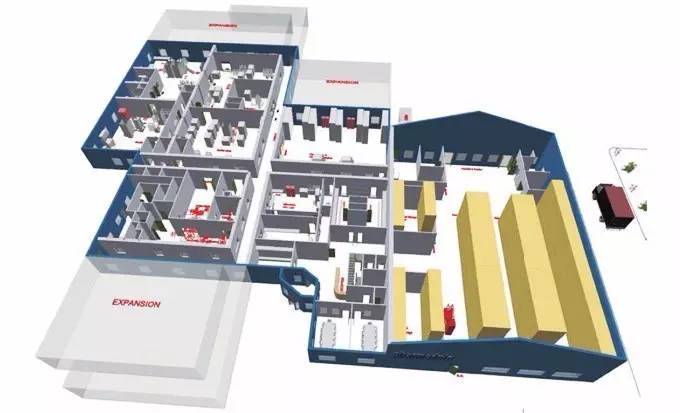
This plant construction method similar to "Building blocks" is fast and efficient. Compared with ordinary projects, modular construction can greatly reduce the manual input, and save 70% of the construction period in the construction phase. The general labor costs account for about 20% of the construction costs, which can directly reduce the project cost by 15%. In the construction process, it can also substantially reduce the construction waste, so that the project can be put into use as soon as possible. After the completion of the plant, the equipment should approach as soon as possible to complete the installation, positioning and commissioning. Therefore, equipment suppliers need to consider how to minimize the installation and commissioning time after the equipment approach to the pharmaceutical plant, so that the plant can enter into the standby for production state at the fastest speed.
02
Equipment Manufacturing
For the pharmaceutical plants that product sterile injections, they also put forward another requirement due to the high value and low yield product characteristics, i.e. to complete the trial-manufacture and production of multiple drugs with the smallest equipment area. This is very attractive from the perspective of cost savings.
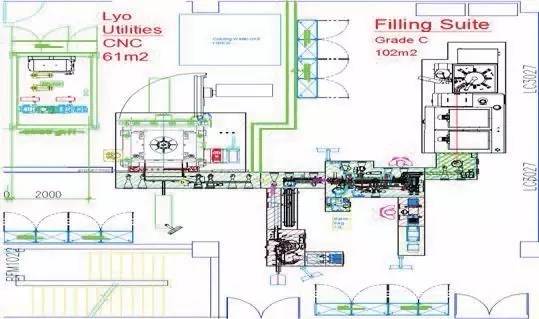
For example, if sterile vials, sterile pre-encapsulation products and sterile card vials can be produced in the same sterile workshop, it will be very versatile as these the packaging methods of the three basically cover the vast majority of sterile injection drugs.
However, in the traditional way, it does not make much sense to place the equipment that produces these three different packaging materials in the same production workshop, because the workshop area occupied by the equipment has not changed, and a large area needs to be sterilized before each production. To reduce the total occupancy area of the sterile workshop, the pharmaceutical plant requires the equipment manufacturers to provide a device that can meet the requirements for the production of the three different forms of packaging materials. At the same time, to reduce the sterile environment grade outside the equipment, pharmaceutical customers require the application of full isolation technology, so that the room sterile environment reaching grade C is acceptable. Hence, the total room area will be reduced to about 30% of the traditional single equipment, and the environment only needs to reach grade C, which can reduce a lot of operating costs.
In response to the above customer needs, Tofflon developed a mixed pre-filling syringe filling line. This is a compact, high-configuration filling line. Three filling and stopper functions for nest plate pre-filling syringes, nest plate card-type bottle syringes and vials are integrated in the same filling line. Changes to the line of production can be achieved through the replacement of moulds. At the same time with the whole set of equipment and completely closed isolator, the equipment can be directly used in class C environment.

In addition, Tofflon also adopted a number of new technologies combined with the actual yield of the customers, such as:
• RTU technology
All packaging materials adopt RTU packaging material, hence saving the vial washing, drier equipment investment and equipment verification work;
• Disposable technology
The combination of peristaltic pump and disposable part (soft bag, hose and filling needle of the filling system all adopt disposable technology) greatly simplifies the batch conversion time of various products without the need for the filling system CIP/SIP, which has better avoided the risk of cross-contamination;
• Robot technology and IPC technology
Through the application of sterile robot technology in IPC (online weighing), minimize the operator intervention in the sterile core area;
• Isolation technology
Through the C + A sterile environment design and the application of isolator technology, greatly simplify the operator's aseptic dressing process, reduce grade A sterile area, which greatly lowers the investment of all resources, reduce the production and operation costs, and achieve the purpose of cost reduction and efficiency improvement for the customers.
03
Software Engineering
If the workshop and equipment are compared to the body of a pharmaceutical plant, the sensors of all equipment are the neural units on the body, and the software system of stand-alone equipment and the entire plant is the brain. To realize efficient and orderly operation of the entire plant, the communication between these systems must be smooth and unimpeded.
Therefore, in the early phase of project design, overall planning is needed for the software system, using the unified communication protocol, unified interface mode and unified architecture. It will determine whether the various workshops and all links can operate effectively and smoothly after the plant is put into production.
Focusing on the creation of the future industry 4.0 smart plant, the automatic control system of the entire production line should be planned in accordance with the MES architecture. Stand-alone control should be achieved in the first phase; central monitoring achieved in the second phase; central control achieved in the third phase, so as to realize the automatic control system that can be rapidly expanded.
With the aforementioned flexible manufacturing characteristics, such pharmaceutical plants are highly reproducible. International multinational pharmaceutical companies can quickly role out the pharmaceutical plant template to their branches all over the world.
Summary
Today, biopharmaceutical industry, especially in the United States, Europe and Japan, is transforming from stable plant to keen plant. Such transformation requires the plants to transform from operating in the familiar external environment to an uncertain and complicated external environment. By providing the flexible manufacturing solution for sterile injections - new technologies and new practices, Tofflon is committed to supporting our customers in the field of biopharmaceuticals, enabling more flexible response of their operation system so that they can achieve success more easily in the face of uncertainty and dynamic market environment changes.