Home > Events >Large-scale Lyophilized Injectable Manufacturing
Large-scale Lyophilized Injectable Manufacturing
The pharmaceutical industry is witnessing major changes and challenges. Today it is growing tendency that global lyophilized generic production is transferring from USA, Europe and Japan to emerging markets and developing countries. Such lyophilized generic production in emerging markets grows up to large-scale lyophilized injectable manufacturing. For aseptic processing of large-scale lyophilized injectables, the challenges and demands we are facing are:
(1) To meet increasing cGMP expectations, especially how to control personnel contamination – the greatest challenge in aseptic critical process;
(2) To keep cost competitiveness and production efficiency as well.
(3) Faster time to market.
To meet the above challenges and demands, Tofflon develops Freeze Drying System for large-scale lyophilized injectables manufacturing. Its typical features are as follows:
Concept
Tofflon keeps paces to the industry trend. We believe that “Automation, Isolation, Continuous Processing and Systems Integration” are becoming main stream for aseptic processing of sterile injectables.
Synergy Global Advanced Technologies
We focus on the critical aseptic process of sterile injectables for the pharma and biotech industry. We synergy global advanced technologies from USA, Europe and Japan. We commit ourselves to make advanced technology more affordable. We always introduce and accelerate cGMP update, latest technologies and best practice for lyophilized injectables manufacturing in dynamic global emerging markets.
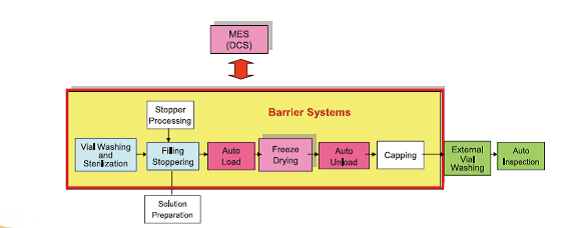
- Lyophilization and Process Knowledge
- Auto Solution Preparation Systems (with Japan Top System)
- Aseptic Vial Filling Line (Italian Technology)
- Freeze Dryer and Auto Loading Systems
- Barrier Systems (with Japan Airex)
- Intelligent Vision Systems (with Italian AVS)
- Automation: Local Control – Central Monitoring – DCS – MES

Systems Thinking and Systems Integration
We work for system Thinking and systems integration both in mechanical integration with upstream and downstream equipments as well as complete process automation integration so as to achieve uninterrupted and consistent process establishment to continuously improve drug quality and production efficiency.
Added Values
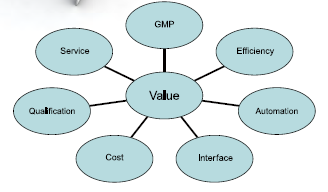
To meet large-scale lyophilized injectables manufacturing challenges and demands, Tofflon Freeze Drying System offers more competitive integrated solution with the following added values:
- cGMP Approach
- High Efficiency
- Automation and Isolation
- Simple Interface
- Cost-Effective
- Easy Validation
- Integrated Service
- Faster Time to Market
Today in emerging markets and developing countries, lyophilized injectable industry is growing towards large-scale lyophilized injectables manufacturing. Tofflon adopts “Automation, Isolation, Continuous Processing and Systems Integration” Concept, synergy global advanced technologies, works for system thinking and systems integration and offers added values towards dynamic emerging markets to meet today’s industrial opportunities and challenges together with pharma and biotech industry.