Home > Events >Mexican Ulsatech Oncology Production Line Project Sharing
Mexican Ulsatech Oncology Production Line Project Sharing
Tofflon Institute Recommendation
In 2014 Tofflon successfully won the Oncology Production Line Project from Mexican Ulsatech. All the critical technology and equipments are from Tofflon, which includes Auto Solution Preparation Systems, Vial Filling Line, Freeze dryer, Auto Loading Systems, Isolator, External Washing Machine and Intelligent Vision Systems.
This is the first oncology production line project in Mexico, even a remarkable project all over Latin America. This oncology production line’s design, manufacturing and qualification are executed as per US FDA and EU GMP guidelines. The oncology products’ OEB level is OEB 5, so the production line shall offer protection for the products as well as protection for the personnel. Tofflon freeze drying system offers an integrated solution from technical design, project management and validation support. This project case study shares with you our latest technology and best practice in freeze drying system for the oncology products.
Tofflon Scope of Supply
- 1 set of Auto Solution Preparation Systems
- 1 set of 100VPM Vial Filling Line, including: Washing Machine, Sterilization Tunnel, Filling Machine, Capping Machine and External Washing Machine
- 2 x Freeze Dryers:LYO-10(SIP,CIP) + LYO-5(SIP,CIP)
- 2 x Fixed (Row-by-Row) Auto Loading Systems
- 1 set of Isolators for Filling Machine, Row-by-Row, Capping Machine, External Washing Machine
- 1 set of API Dispensing Isolator for Auto Solution Preparation Systems
- 1 set of Intelligent Vision Systems for liquid vials and lyophilized vials auto inspection
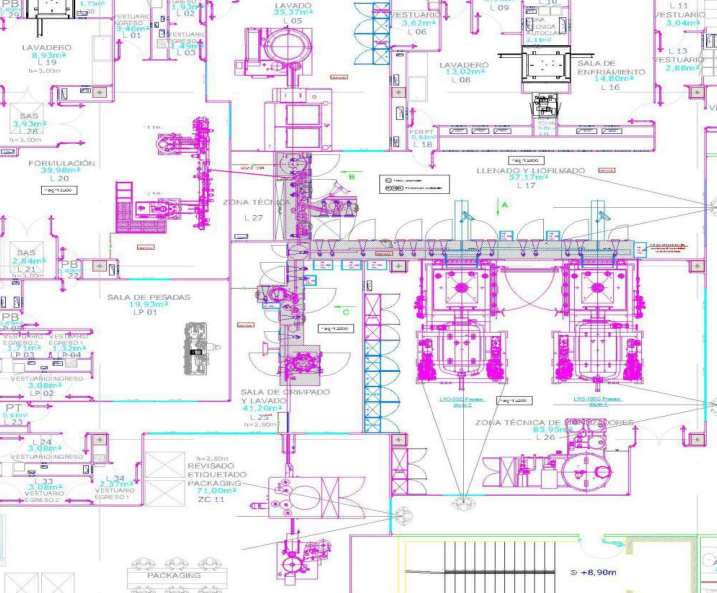
In this project, Tofflon Freeze Drying System offers integrated solution. Upstream and downstream equipments can be well integrated which much reduces the complicated interfaces risk from different vendors machines. Meanwhile the whole facility can be well tested and qualified as one complete module to ensure each machine’s capacity can match each other so as to avoid possible machine’s down and idle time.
“T” layout design can meet both liquid vials and lyophilized vials production. The buffer turntable can shunt the liquid vials and lyophilized vials production flow.
Clean room adopts the visible glass wall. It offers comfortable visualization for visitor and inspectors.
Control system: English and Spanish version can be chosen. It greatly makes the whole operation and maintenance job more friendly and convenient for local operator.
Central Monitoring System allows facility supervisor check all machines running status in one system. If there is any alarm, machine breakdown or operator’s incorrect operation, this system can easily display and record the event. It can achieve central monitoring, central traceability and central problem analysis.
Siemens PLC modular is adopted for each individual equipment communication on the uniform platform to increase the efficiency and to reduce the programming time.
Central deactivation and individual deactivation design: Each toxic liquid leakage point can be individually deactivated by spraying deactivation liquid and neutralization liquid or the operator can collect all the toxic liquid to the central deactivation system for final deactivation.
Part 1: Company Introduction
Ulsatech SA DE CV is a Mexican company located in Guadalajala City. The plant is spread over an area of 2,000 square meters, with a built up area of over 5,000 square meters. The equipments, utilities, plant and machinery meet the international standards. It has a well-equipped analytical and microbial testing facility. The manufacturing facility works for the highest possible quality standards of Mexican, US and European standards.
Part 2: Project Introduction
Its annual capacity is 20 million vials. As the first Mexican oncology plant, it breaks through multinational pharma companies’ monopoly on high value oncology drugs. The products are not only sold in Mexican market, but also exported to Middle American countries and North American markets. Therefore all the facility shall meet Mexican and international standards. Considering high toxic level, so the facility’s deactivation and isolation will be validated by the professional US company.
Part 3: Key Technology
1. Auto Solution Preparation Systems
2. Vial Filling Line
3. Isolator
4. Freeze Drying System
5. Intelligent Vision Systems
We believe that “Automation, Isolation, Continuous Processing and Systems Integration” are becoming main stream for aseptic processing of lyophilized injectables. Mexican Ulsatech Oncology Production Line shares our latest technology and our recent best practice under this technical concept. We do hope it offers valuable information for your cGMP practice.