Home > Events >Flexible Lyophilized Injectable Manufacturing
Flexible Lyophilized Injectable Manufacturing
Flexible Lyophilized Injectable
Manufacturing
It has been widely
recognized that the global pharma industry is currently experiencing dynamic
change, especially for biotech industry. Withers
& Rogers’ study in particular bolsters the paradigm that big pharma is
shifting its development focus from small molecule chemicals to large molecule
biologics in recent years.
And the
personalized medicine requires ever more flexible and versatile processing and
packaging solutions. Smaller batch sizes shift the emphasis from speed and mass
production of standard dosage products to more individualized products packaged
in high-quality materials. Short start-up times, easy changeovers and a high
degree of automation are key considerations. Meanwhile pharmaceutical manufacturers
are now expected to quickly produce a wide variety of products at significantly
lower volumes. These changes have placed increased pressure on drug makers to
accelerate product development while reducing development costs.
These new drivers are pushing many
pharmaceutical companies to look beyond traditional batch manufacturing
processes and begin experimenting with a variety of continuous and flexible
manufacturing techniques. Flexible Lyophilized Injectable
Manufacturing has the following typical features: high value, low volume,
automation and isolation, flexible production line for multiple containers and
multiple products, ready-to-use technology and disposable technology are widely
applied.
At Achema Frankfurt 2015, Tofflon shows its latest developed Combo Filling Line for Flexible
Lyophilized Injectable Manufacturing. Its key features are as below:
(1) Flexible manufacturing: syringes,
cartridges, liquid vials and lyo vials combined in one line;
(2) RTU Technology;
(3) Single Use Technology;
(4) In-process Control (IPC) Technology;
(5) Isolator Technology;
(6) Expandable Automation Design;
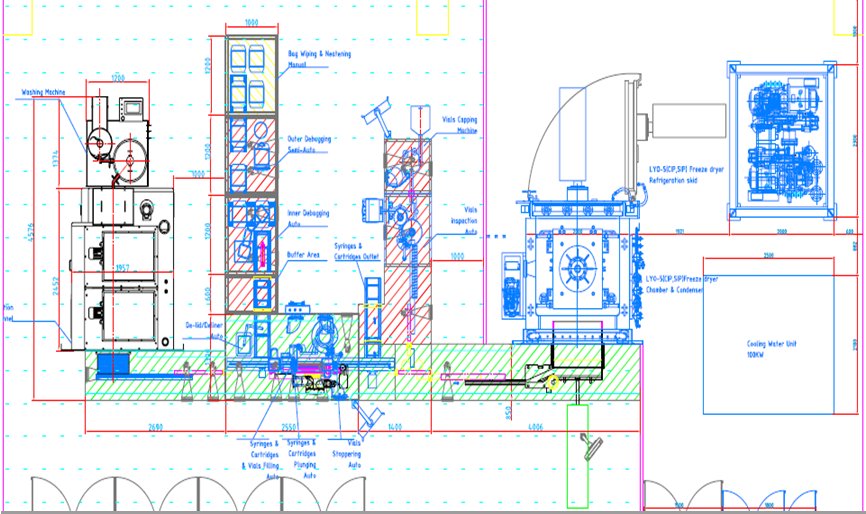
Flexible manufacturing
The Combo Filling Line is capable for
multiple containers and multiple products with rapid changeovers. It can be
flexible for syringes, cartridges, liquid vials and lyo vials in one line. For
syringes/cartridges, their processing includes semi-automatic de-bagging, automatic
de-lid/de-liner, In-Process-Control (IPC), aseptic filling with peristaltic
pump and single-use disposable components, stopper plugging with vacuum insertion.
For liquid vials/lyo vials, their processing includes bulk vials washing with
rotary washer, sterilization with sterilization tunnel, In-Process-Control
(IPC), aseptic filing with the same peristaltic pump and single-use disposable components,
half or full stoppering, capping with capper. In case of lyo vials, their
processing also includes fixed (Row-by-Row) auto loading and unloading, lyophilization
and full stoppering.
RTU Technology
Today ready to use (RTU) glass containers are being developed for pharmaceutical
use. It has been applied to clean, sterile, depyrogenated and ready to fill
vials, cartridges and syringes in a next & tub format. The solution allows pharmaceutical
companies to reduce upstream operations linked to containers preparation,
sterilization and validation thanks to a lean, modular and smart solution that
improves efficiency.

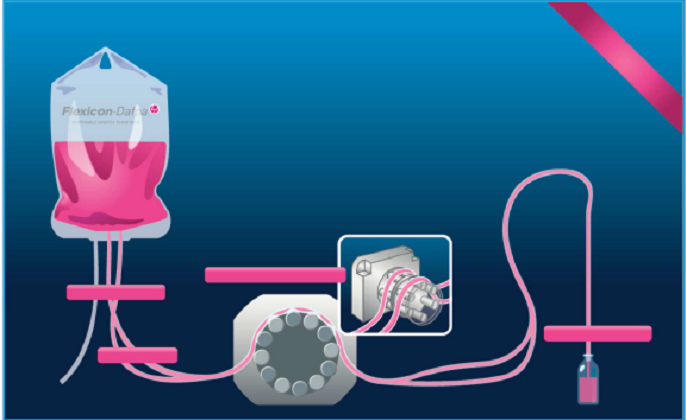
Single Use Technology
The early biopharmaceutical production
line is designed to manufacture a single product and all hard piping is with
portable or disposable equipments. Tofflon Combo Filling Line widely combines
the single use technology during the production .With the shift to smaller
batches, the increase of use highly potent substances and highly industry
safety regulations, there rises up the use of single-use, pre-validated,
pre-assembled and pre-sterilized components including hoses, buffer bag,
filling needles and tubing to improve the production efficiency and capacity
without the required time-consuming CIP/SIP throughout the process between
batches. The single use technology largely minimizes the risk of contamination
between batches run.
In-process Control (IPC) Technology
To improve In-Process Control and reduce
high-value products loss, In-Process Control (IPC) Technology is applied for
both syringes/cartridges and liquid/lyo vials. At the beginning and end of one
batch, 100% IPC will be requested. In the middle of production, statistical IPC
will be applied.
Isolator Technology
Current Good Manufacturing Practice (cGMP) Guidelines
require dedicated facilities to minimize the risk of contamination. Tofflon Combo
Filling Line is designed with integrated barrier systems [Active oRABS, cRABS
and Aseptic and (or) Toxic Isolator].

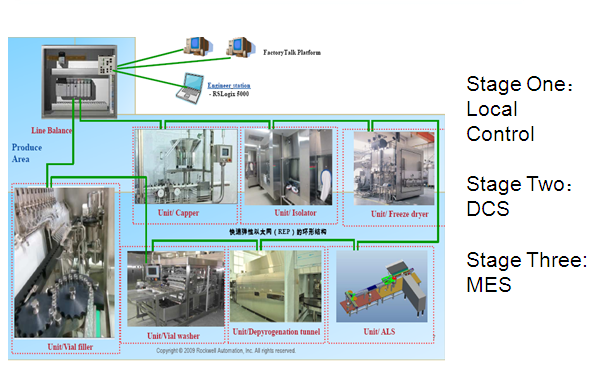
Expandable Automation Design
As part of Flexible Manufacturing Model,
Tofflon adopts “Expandable Factory Design Concept” - rapidly add modules as
needed. Presently “Just Enough” and In the future “Just-in-time” to add modules
as needed. Therefore Tofflon Combo Filling Line offers expandable automation design:
At Stage One: Automation based on Local Control. Stage Two: Local Control can
be rapidly upgraded to “DCS”. In the future Stage Three it can be further built
as part of MES.