Home > Events >Visual Inspection of Parenteral Products Part 8 Inspection of Different Kinds of Products / Containers
Visual Inspection of Parenteral Products Part 8 Inspection of Different Kinds of Products / Containers
|
Visual Inspection of Parenteral Products Part 8 Inspection
of Different Kinds of roducts / Containers |
In the previous parts we discussed inspection of vials and
ampoules because they are the most important containers by production numbers
and because they were the first to be inspected by automatic inspection
machines.
Even though more than 90% of automatic inspection systems
are today sold and installed for such containers, we can't forget the wide
range of other pharmaceutical containers which naturally require visual
inspection and Quality Control as well.
As we will see, the main point is that the features of
other containers make it more difficult to achieve an effective automatic
inspection and this has slowed down the development and diffusion of automatic
inspection for such kind of containers while manufacturers continue to rely
more on manual inspection. Nonetheless we can be sure than this gap will
gradually disappear because the same push to introduce automatic inspection and
its advantages (higher speed, more stable and repeatable inspection level, much
better data collection and precision) is there and newer technologies and
designs become year after year available to solve technical problems.
Lyophilized Products (Lyo Cake)
According to most regulations, even lyophilized products must undergo the same 100% inspection as liquid products. However, after the freeze-drying and the capping process, the solid lyophilized cake becomes not transparent and the presence of particles can be detected only when they are located on the external visible surface. Furthermore to be effectively detected, particles must provide sufficient contrast with the lyo solid matrix, which usually is white or light yellowish color, because detection cannot be based on movement of particle such as in the case of liquid.
Because the external surface of the cake visible during
inspection accounts for only a small fraction of the cake bulk volume there is
a much higher probability of non-detection of non-visible foreign particles
compared to liquids.

Since this is a destructive inspection, it must be limited
to a sample set of every batch. As per liquid products AQL check, sampling
according to ANSI/ASQ Z1.4 plans can be used.
If particles are detected in this relatively small sample,
it is recommended to reconstitute more samples in order to investigate the
overall quality and compliance of the whole batch.
Dry Powder Products
Another common problem for powder products is when during container handling some powder ends up sticking to the inner sidewall of the container, reducing even further the inspection possibility.
Similarly, as described for lyo, sterile powders should also be sampled, reconstituted and inspected for visible foreign particles.

Automatic Inspection of F/D And Powder
Products
With the development of newer and better automatic visual
inspection technologies, several AVI machines have been gradually developed and
introduced to inspect freeze dried and powder filled containers.
The main approach is to replicate the manual inspection by
inspecting with several cameras the whole external surface of the cake or the
powder looking for foreign particles.
Using this approach, the same automatic inspection machine
is able to inspect both freeze dried and powder filled containers.
Despite the additional level of sophistication of these inspection systems, AVI for freeze dried and powder products are becoming more and more common because they have to be compared in performances to Manual inspection which has basically the same troubles and limitations with this class of products.

Emulsions and Suspensions Products
Other important categories of products where basically
only the outer surface of content is visible for inspection are suspensions and
emulsions.

X-rays
An alternative approach to inspect non-transparent
products has been based on X-rays characteristic to travel through the matter.
However, X-rays have some intrinsic limitations which limited the success and diffusion of this kind of machines: detection is basically possible only with foreign materials with density very different from product's and there are concerns about the safety of the operators working around the machine and the product itself.

Red/Dark/Amber Containers or Liquids
Manual Inspection of products contained into dark/amber
containers or in red/dark is challenging.
Since these containers are still partially transparent to
lower frequencies of light (usually red and near-infrared) higher brightness is
recommended for manual inspection (8-10.000 lux instead of 2-3750).
On the contrary, in this context, automatic inspection is greatly advantaged and more efficient because cameras, lights and lenses can be optimized to work with the highest resolution and sensitivity in the region of the spectrum where the glass&product are still most transparent. So with this kind of product automatic inspection can operate well and virtually unaffected in performances by dark color.
Translucent
Plastic Containers / Blow Fill Seal (BFS)
Plastic translucent containers are more and more
commonly used to compensate disadvantages of glass
(easy to break, heavy, needs high temperature sterilization, etc.).
Similarly, to
dark/amber containers, plastic is generally much less transparent of glass for
visual inspection and therefore manual inspection is not so effective on this
kind of products.
Once again, higher
brightness is recommended for manual inspection as well as optimization of the
lighting setup in accordance to the characteristics of the container.
Even in this case, automatic
inspection systems can be better optimized to provide the best resolution and
inspection efficiency of these containers, not only in the light and imaging
part, but also in the processing software to compensate the lower transparency
and contrast caused by the plastic container.

Large-Volume Containers (LVP)

In case of plastic containers, we have to add as
negative factor we saw in the BFS category the reduced transparency of the
container sidewall which reduces the ability to detect small particles and
illuminate properly the liquid inside.
Despite of these extra-difficulties, automatic inspection machines have been available for this kind of products already for quite a long time and though optimizations and careful design have reached a level of performances generally equal or superior to manual inspection. Their main disadvantages compared to AVI for smaller volume are the bigger size of machine, higher cost and reduced inspection speed.
Soft-bags
The
inspection of plastic soft bags can be considered as an extension of LVP
containers with the addition of more difficult handling and imaging caused by
their shape and construction.
Finally,
handling and spinning them for inspection is quite difficult.
Because of all these issues, automatic inspection of soft bags is still very limited and can be considered one of new field of application for future automatic inspection machines.

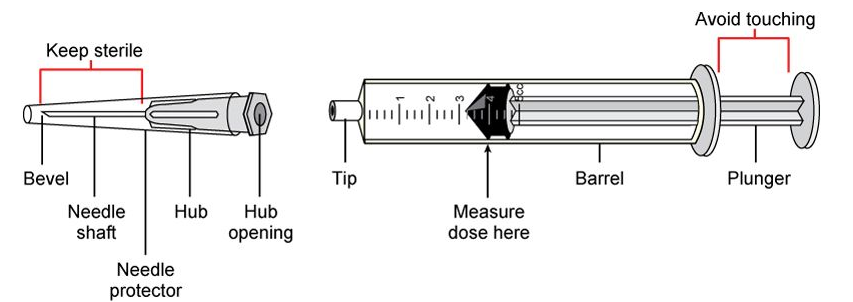
Pre-filled Syringes (PFS)
The last family of containers to be inspected
for injection products is PFS.
This is a rather new sector, where development
of automatic processing machines has started only recently and consequently
also regulations aren't yet complete nor clear.
In theory they are subject to similar
requirements for foreign particles, filling level, capping and container
closure system as other containers we checked before, so most manufacturers and
designers of automatic inspection machines are trying to mimic the same range
of controls.
Because of this, there is a rather wide range of
automatic inspection systems already available for PFS heavily customized where
controls and specifications also change a lot between machine and machine.
We can consider this field together with IV Soft bags a frontier area for Visual Inspection, where in the future we will see regulators and suppliers of machine work to define, regulate and develop standard criteria and inspection systems for Quality Control.