Home > Events >Brief Analysis on Blow-Fill-Seal (BFS) and Its Applications
Brief Analysis on Blow-Fill-Seal (BFS) and Its Applications

BFS sterile filling production line, which consists of a set of sterile
preparation systems and a BFS sterile filling machine, is a relatively complete
sterile filling production line. This production line has the full-line CIP,
SIP functions and works under the protection of positive pressure, air
tightness and laminar flow. Container manufacturing, product filling, and
container sealing (blow – fill -seal) are all done under the sterile protection
of a Class-A air shower, which can effectively prevent all kinds of pollution
and cross contamination that may occur during the process. Temperature,
pressure, time / speed and other process parameters during the process can be
monitored online and recorded in real time, which fully meets the requirements
of sterile process. As the BFS sterile filling production line has strong
sterility guarantee ability, no preservatives need to be added to products,
which can achieve “non-final sterilization”. What’s more, products are of high
inherent quality and safety.
Process Layout of BFS Sterile Filling Production Line
Both types of devices can meet the requirements of sterile filling. BFS has two layouts: black and white partitioned layout
and non-partitioned layout.
The black and white non-partitioned layout integrates some motors, hydraulic pressure, pumps and the like of the device under the device, so it’s relatively small. Its disadvantage is that particles will be generated during the work of these parts, which will cause the particles in the room to be high. Besides, the device parts are stacked too tightly, which will cause inconvenience to subsequent daily maintenance.
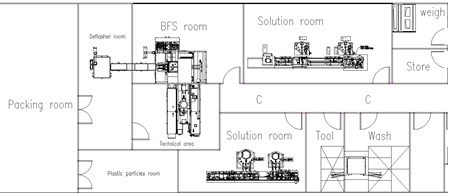
BFS Layout
In the BFS production process, although the plastic granule silo can be
placed in the general area (control clean area), the pollution prevention of
plastic granules is also very important. It is recommended to store the silo
in a separate small room and use a dust-proof hopper with a lid. Except for
regular feeding, personnel should not stay in the room to avoid inhaling dust
and foreign materials.
Sterile Management in the Production Process of the BFS Device
For this reason, it is necessary to monitor the key areas of the device and
deal with it in time when the device breaks down.
In the CIP / SIP process, great attention should be paid to the effect of
cleaning and sterilization and the integrity test of each filter element.
Replace hoses and seals regularly, perform leak detection on pipeline valves,
and find damaged hoses and seals in time. When the dosing system transfers the
chemical liquid to the BFS system, it is recommended to use a pressure pump or
an easy-to-clean pump. When the product is suspension, whether the hardness of
the liquid granules will affect the mechanical seal of the pump should be
considered. Measures of isolation and back pressure elimination should be taken
at the main drain to prevent back pressure in the drainage pipeline from
causing unqualified BFS cleaning and sterilization and reverse pollution.
As the BFS filling area isolates the pollution in the room by a positive
pressure air curtain, the sterility detection and control of Class-A air shower
should be done. In addition to the various data measurements, attention should
also be paid to the status of the Class-A fan and whether its blades and
bearings are abraded and have debris, which may damage the filter element, and
the good working condition of the fan can also greatly reduce the load of the
Class-A filter element.
Application of BFS in the Production of Sterile Drugs

Application of BFS in Food and Cosmetics Production
BFS sterile filling technology was initially applied to sterile food production. It’s currently limited by the production process in the domestic market. Many functional foods and cosmetics are not produced by the sterile process, so they all contain preservative ingredients to avoid spoilage. The preservative principles of preservatives are to prevent or eliminate microbial contamination, inhibit the growth and metabolism of microorganisms, and kill microorganisms. In terms of the preservative principles of preservatives, preservatives are harmful to human tissues. Food and cosmetics produced by the BFS sterile process do not need preservatives. It is particularly suitable for the production of plant extracts with homology of medicine and food, such as ginseng extracts; single-use small packages of sterile cosmetics are accurately measured and easy to use and carry and can also prevent pollution and cross contamination during use, such as various extracts and sterile masks in cosmetics.As some cosmetics and foods are relatively thick and cannot be sterilized and filtered, before products enter the BFS device, appropriate methods should be used to ensure their sterility.

In short, BFS sterile filling process is currently recognized as an
energy-saving and environmentally friendly sterile filling process with strong
sterile guarantee ability, high technical content, production efficiency and
product added value. The special functions of BFS sterile filling process and
products determine that it will gradually replace the traditional “wash-fill-
seal process” and become the inevitable development trend in production of
sterile products.