Home > Events >
Probiotics is essential in human’s daily life, and probiotic products in solid form are generally produced by freeze-drying/lyophilization.
The main quality indicator for probiotic products is the quantity of live bacteria during the validity period. Tofflon is dedicated to exploring
the maximization and automation of probiotics production capacity and efficiency while ensuring quality indicators.
General process for the production of freeze-dried probiotics
The general process for the production of freeze-dried probiotics is as follows:
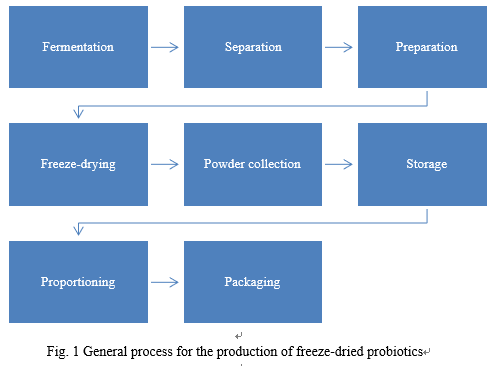
A good probiotic strain is identified for culture and screening, and the fermentation is scaled up to the industrial scale through the fermenter.
-
The bacterial
sludge is mixed with the protectant in a preparation tank to obtain a
suspension containing probiotics.
-
The
suspension is filled on the lyophilizer shelf, freezing, vacuuming, heating and
drying on the shelf until the endpoint is reached. The lyophilizer restores to
normal pressure. Open the door to remove the freeze-dried powder.
-
The
freeze-dried powder is collected into a closed container and
-
placed in
cold storage for storage.
-
When
proportioning production is needed, the stored bacterial powder bin is removed
from the cold storage for quantitative feeding, proportioning and mixing
through the proportioning and mixing system. After the mixing is processed, the
proportioning bin is connected to the packing machine for the dispensing of
finished products. Eventually, the dispensed products are packaged and then
released in the market.
-
The bacterial
sludge is mixed with the protectant in a preparation tank to obtain a
suspension containing probiotics.